CA CURE GRANTS
Committee to Advance the Understanding of High-Risk GI Cancers
Our newest grant initiative, CA CURE, aims to fund research to make advances in the understanding, diagnosis, and treatment of gastrointestinal cancers.
CA CURE Grant Awards
Despite centuries of research, treating and curing cancer remains an urgent health research priority. Far too many people at younger and younger ages are diagnosed with fatal cancers, often after living with a digestive disease such as Crohn’s, ulcerative colitis, fatty liver disease, and others. Despite its prevalence, colon cancer research is grossly underfunded.
With extraordinary and transformational support from anonymous donors, the GI Research Foundation launched a bold initiative, CA CURE, to identify and fund research to improve diagnostics and develop immunotherapies and personalized vaccines.
Beginning in 2022, CA CURE sought projects that might have difficulty attracting funds because they are too experimental or are in the initial stages of development. With a focus on improving patient outcomes, we funded nine rigorously evaluated research projects from across the country. In its initial phase, CA CURE put vital research dollars in the hands of leading scientists. Thanks to the expert scientific review from eight physician-scientists and investigators at the University of Chicago, Yale University Medicine, Weill Cornell Medicine, and MD Anderson, the GI Research Foundation developed a scientifically robust review process.
In just fourteen months, the Foundation awarded $22,189,567 in grants.
This has been an extraordinary team effort, demanding time, expertise, and wisdom from many. The GI Research Foundation is so fortunate to have scientific leaders who care about our success and lay leaders with the passion to immerse themselves in learning complicated research.
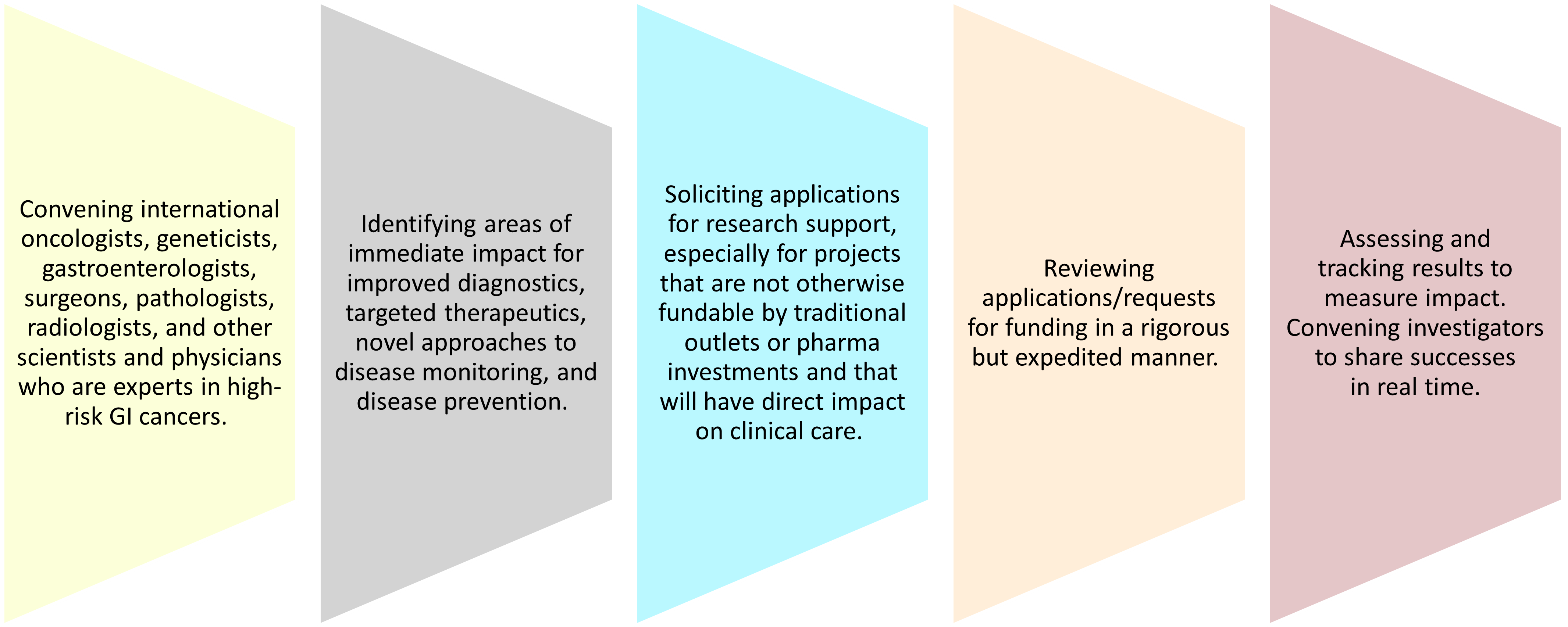
The Charge of CA CURE
Committee to AdvanCe the Understanding of High-Risk GI CancErs
Funded Projects
Image-Guided Histotripsy for Treatment of Colorectal Liver Metastases
Osman Ahmed, MD, FCIRSE
University of Chicago
$500,000 award
Recently, a completely non-invasive technology, histotripsy, has emerged that uses ultrasound to destroy tumors without heat, needles, radiation, or incisions. This approach reduces the theoretical risks of bleeding associated with invasive or “minimally invasive” procedures and allows for multiple recurring treatments. The purpose of this study is to investigate histotripsy in patients with metastatic colon cancer to the liver during chemotherapy. Documenting the safety of combined treatments is the first critical step in evaluating improvements in outcomes.
Development of a Lymph Node Targeting Vaccine for Patients with BRAF V600E and TP53 R248W Tumor Neoantigens
$2,756,000 award
Complete GMP Manufacturing, Regulatory Interactions, and Clinical Preparations to Enable Single-Patient Clinical Assessment of AMP-Peptide Vaccine Therapy Targeting BRAFV600E and TP53R248W
Robert Connelly, CEO
Elicio Therapeutics, Inc.
$2,602,596 award
Elicio’s research project seeks to develop two therapeutic cancer vaccines. Both vaccines have been designed with Elicio’s proprietary lymph node-targeting Amphiphile (AMP) platform that “educates” T cells on how to target particular antigens, such as mutated proteins in cancer.
Colorectal Cancer (CRC) Cellular Heterogeneity, Metastasis, and a Spatially-resolved Molecular Atlas for the Colon
Christopher Mason, PhD
Weill Cornell Medicine
$833,515 award
Funding to Dr. Christopher Mason at Weill Cornell Medicine (WCM) will support research utilizing a cutting-edge, sub-cellular spatial profiling technology to reveal novel aspects of colorectal cancer (CRC) heterogeneity and improve our understanding of normal tissues, at both the RNA and protein levels.
Advanced Approaches to Understanding and Targeting BRAF Tumors
Scott Kopetz, MD, PhD
MD Anderson Cancer Center
$3,519,021 award
With GI Research Foundation support, MD Anderson will conduct three interrelated projects designed to improve survival in patients with BRAF-mutated (BRAFmut) colorectal cancer (CRC), moving promising laboratory findings into new clinical trials to develop novel treatment approaches in the clinic.
CTC Sample Collection, Processing and Biomarker Testing
Leighton Howells, Senior VP
RareCyte, Inc.
$1,065,200 award
Rarecyte has been funded to test gastro-intestinal cancer patient samples within clinical trials to determine circulating tumor cell (CTC) burden and selected biomarker analysis. The emerging trend of personalized medicine (‘patient specific therapy’) requires deeper understanding of the makeup of CTCs, both at the protein and gene level, to select therapies which will specifically treat the individual patient’s cancer.
Novel Individualized Therapeutic Strategies for Metastatic Colorectal Cancers
John A. Copland, PhD
Mayo Clinic
$5,942,244 award
This project proposes three different strategies to enhance survival and potentially cure cancer—optimization of the immune system, activation of the immune system to combat cancer, novel combination therapies. Two strategies are in the human clinical trial phase, and one is in the discovery phase.
Development and Optimization of Targeted Drug Formulations for Colon Cancer
Aliasger K. Salem, PhD
University of Iowa
$1,698,949 award
Colorectal cancer presenting with rare combinations of multiple mutations and demonstrating resistance to standard therapies, requires novel multipronged therapeutic strategies that supersede current conventional approaches. The University of Iowa will test the effect of combining specific drug combinations to target colorectal cancer cells possessing the relevant mutations, delivered in innovative ways.
Development of 5hmc Based Plasma Signatures (Liquid Biopsy) in the Detection of Patients with Peritoneal Metastases
Kiran Turaga, MD
Yale University School of Medicine
in collaboration with the University of Chicago Medicine
$1,382,041 award
Cancers of the colon, rectum and appendix can spread to the lining of the abdominal cavity and are difficult to detect. Measurement of DNA released by cancer cells is a novel new technology that is being applied to diagnose cancer spread to the lining. However, conventional methods of DNA measurement have low sensitivity to identify this spread. This project, a collaboration with the UChicago Medicine, will harness novel technology developed there (He lab) to detect these cancers early by using exciting novel approaches of liquid biopsy.

A Single Arm Phase II Study with Safety Run-In of Peptide Receptor Radionuclide Therapy (PRRT) in Combination with Immunotherapy for Patients with Merkel Cell Cancer
Pashtoon M. Kasi, MD, MS
Hoosier Cancer Research Network
$1,100,000 award
This project proposes three different strategies to enhance survival and potentially cure cancer—optimization of the immune system, activation of the immune system to combat cancer, novel combination therapies. Two strategies are in the human clinical trial phase, and one is in the discovery phase.

Capturing Circulating Tumor Cells as Liquid Biopsies for Patients with Advanced/Metastatic Colorectal Cancer and Other Malignancies
Pashtoon M. Kasi, MD, MS
Weill Cornell Medicine
$290,000 award
Liquid biopsies are revolutionizing cancer care. This project focuses on circulating tumor cells (CTCs), where little research has been done to date. Focus on CTCs might allow capture of intact cancer cells that can be used for myriad of biomarker testing that cannot be done on plasma ctDNA. When processed appropriately, they can be noninvasively cultured to develop cell lines and organoids for use in treatment, which now require tissue from a generous biopsy and/or surgery, which isn’t always safe or feasible.
CA CURE Leadership

David T. Rubin, MD
University of Chicago Medicine
Senior Scientific Advisor, GI Research Foundation Chair, CA CURE

Yekaterina Chudnovsky
Chairperson, GI Research Foundation
CA CURE Scientific Review Panel

Scott Kopetz, MD, PhD
MD Anderson Cancer Center
The University of Texas

Pashtoon Kasi, MD, MS
Weill Cornell Medicine
Cornell University

Kiran Turaga, MD
Yale School of Medicine
University of Chicago Medicine

Christine Drogan, MS
Genetic Counselor
Section of Gastroenterology, University of Chicago

Christopher Weber, MD, PhD
University of Chicago Medicine

Christopher Mason, PhD
Weill Cornell Medicine
Cornell University
Join Us
Learn how you can actively support our mission to prevent, treat, and cure digestive diseases.

